生物成像和基因技术的快速发展,给生命科学带来了革命性的变化。自2010年北京大学生物动态光学成像中心(2018年更名为北京大学生物医学前沿创新中心,BIOPIC)成立以来,跨学科科学家们致力于发展前沿生物技术,并利用这些技术去解决生物学的基础问题和威胁人民健康的医学问题。中心的研究成果提高了人们对生物系统、传染性疾病和非传染性疾病的认识。在BIOPIC成立十周年之际(点击查看详情:http://news.pku.edu.cn/xwzh/0c4228c25ed94bdf85621379b17cdd98.htm),其创始人、单分子生物学领域先驱谢晓亮回顾了中心的发展历程,并分享了他关于单细胞技术发展的愿景。

是什么原因促使您成立BIOPIC?
我的学术生涯以生物物理化学家起步,因为我喜欢操作仪器。物理化学为理解生物分子过程提供了工具,推动实现了许多生命科学研究的进步。最初,我专注于生物成像,使用单分子荧光显微镜和其他工具,监测、分析和操纵活细胞中单分子的活动。这些工具改变了生物问题的研究方式:科学家们可以在不破坏生命体正常生理状态的情况下研究单个分子的活动。
随着下一代测序技术的发展,科学家们能够以更快的速度、更低的成本完成基因组分析。这场生物科学领域的技术革命,带来了精准医学的新时代。看到新型测序技术的应用潜力,我开始转向单细胞基因组学,并开发了属于团队自己的测序仪。同时,我萌生了建立一个中心,专门研究基础科学和开发医学前沿技术的想法。我和另外两位北大校友——苏晓东教授和黄岩谊教授向母校提出了这个想法,于是2010年,BIOPIC正式成立。
BIOPIC为何与众不同?
BIOPIC是一个技术驱动的生物医学研究中心,集基础研究、技术研发和临床应用于一体。我认为研究工具的突破和跨学科的整合对推动生命科学研究的发展至关重要。为此,BIOPIC引进了拥有多学科背景的研究人员,包括我这样的物理化学学家或生物物理学家,还有结构生物学家、分子生物学家,以及从事生物技术或工程、数学,和计算科学的研究人员。中心的很多突破性的生物技术都是跨学科合作的成果。我认为,BIOPIC的跨学科水平是数一数二的。
强调临床转化也是BIOPIC的独特之处。BIOPIC虽有很多科研成果发表在顶级学术期刊上,但这不是中心师生的最终目标。BIOPIC鼓励研究人员进行技术产业化,与临床医生合作,将技术应用至临床转化。BIOPIC的科学家们有志于推动基础科学的发展,也期望运用我们的技术造福社会。
BIOPIC关注的前沿技术有哪些?
最初,基于我早年间在哈佛大学的单分子成像工作,BIOPIC被命名为生物动态光学成像中心。如今中心的研究范围大为拓展。BIOPIC专注于单细胞基因组学技术,包括高通量测序、基因编辑和微流控。在大数据分析的加持下,这些技术加上单分子成像、超分辨率和无标记成像技术,可以用于推进基础科学研究——从基因组学、遗传学和分子生物学,到发育生物学、肿瘤免疫学和生物信息学。BIOPIC也重视技术的临床转化。BIOPIC开发的全基因组扩增技术可以实现高覆盖率的测序,并已用于胚胎植入前基因筛选。BIOPIC师生们研发的纠错码测序、基于CRISPR的测序,以及一种安全、高效的基因编辑工具,已经用于分析人类胚胎发育、干细胞和肿瘤微环境,为生殖医学、癌症和传染病的诊疗提供参考。

您为什么聚焦单细胞基因组学?
单分子技术使科学家们能够在分子水平上解释生命过程,这是20世纪的一项重要进展。单细胞基因组学是一种下一代测序技术,有可能带来一场更大的革命。我相信,其影响比人类基因组计划更大。它能帮助科学家们探究细胞内存在的全部DNA和RNA,绘制基因组结构和功能图谱,回答DNA或RNA携带的遗传信息如何控制细胞功能等问题。这将提供对于一些基础生命科学问题的新认知,并为临床应用带来新的可能性,包括产前基因检测和癌症诊断等。
要想在这个快速发展的领域站稳脚跟,中国必须发展单细胞基因组学技术,包括研发知识产权完全属于自己的测序仪。而BIOPIC正在推动这样的技术发展。
单细胞技术如何为精准医学铺路?
精准医学必须考虑到基因的个体差异性。单细胞基因组学能够加深医生对疾病的遗传学理解,确定最适合个体的治疗方法。例如在癌症治疗方面,利用下一代测序技术,医生可以分析单个肿瘤细胞及其微环境,确定新的治疗靶标。最近,BIOPIC的科学家利用单细胞RNA测序技术揭示了肝癌中单个免疫细胞的动态,为开发潜在的治疗策略带来了启示。
此外,BIOPIC的研究团队与北京大学第三医院的乔杰院长合作,提高了胚胎植入前的基因诊断和筛查水平。基于早期开发的单细胞全基因组扩增技术,BIOPIC研发的新方法已被用于从已知患有单基因遗传病的夫妇中,高精度地选择健康胚胎。
BIOPIC如何促进研究转化?
BIOPIC的目标是将技术转化为应用。转化研究需要一个健康流畅的生态系统,北京大学在各个基础领域都有丰富的资源和优势。临床上,北大有附属医院;地理位置上,北大离中关村科技园区的生物中心也足够近,这里有生物技术巨头(如百济神州),也有许多初创企业。BIOPIC的研究人员们已经创办了6家生物技术公司,其中有5家是在北京注册的。BIOPIC为科学家们提供足够的支持和发展空间,让他们敢想敢做,同时也扶持了一个关系紧密的校内-校外共同体,让科学家之间的协同作用激发出各种奇思妙想,并转化为临床成果,服务于整个社会。
您对BIOPIC的期望是什么?
我们的目标是BIOPIC成为生物医学领域世界领先的研究机构,推动前沿生物技术的原创性研究,开发和利用创新的生物成像与基因技术,为在分子和细胞水平上探索基础生命科学和医学研究等问题提供工具。我也期望BIOPIC的工作能够转化为技术和医学上的突破,最终造福社会和全人类。

BIOPIC PI(左起:孙育杰、白凡、高歌、黄岩谊、张泽民、邓伍兰、魏文胜、谢晓亮、汤富酬、葛颢、苏晓东、邢栋、高毅勤、赵新生)
附:Nature特刊——Biomedical Pioneering Innovation Centre, BIOPIC: A leader in single-cell technologies

A technology-driven development strategy defines the success of Peking University’s Biomedical Pioneering Innovation Centre (BIOPIC). Since its founding in 2010, it has been dedicated to developing cutting-edge bioimaging and sequencing technologies, which have revolutionized biological sciences. In addition to driving fundamental biological research, their technologies have also been harnessed to tackle compelling medical problems, ultimately benefiting society.
Decoding biotech success
Rapid developments in bioimaging and sequencing technologies have revolutionized biological science. Since Peking University’s (PKU) Biomedical Pioneering Innovation Centre (BIOPIC) was founded in 2010, an interdisciplinary group of researchers has been dedicated to developing these cutting-edge techniques, and harnessing them to address fundamental biological issues and compelling medical problems. Their results have improved understanding of biological systems, as well as infectious and non-communicable diseases. On the 10th anniversary of BIOPIC, its founding director, Xiaoliang Sunney Xie, a pioneer in single molecule biology, reflects on its growth, and shares his vision for the development of single-cell technologies.

Xiaoliang Sunney Xie, BIOPIC’s Founding, Director Credit: BIOPIC
What led you to establish BIOPIC?
I started my academic career as a biophysical chemist, as I enjoy playing with instruments. Physical chemistry has offered tools for better understanding biomolecular processes, fuelling many advances. I originally focused on bioimaging, using single-molecule fluorescence microscopy and other tools, which allow monitoring, analysing and manipulating activities of single molecules in live cells. It changed the way biological problems are addressed.
As next-generation sequencing takes off, genomic analysis can be done much faster and more cheaply. This technological revolution in biological sciences is bringing a new era of precision medicine. Seeing the application potential of new sequencing technologies, I began to shift to single-cell genomics, and developed our own sequencer. Meanwhile, the idea of building a centre dedicated to developing and harnessing cutting-edge technologies for fundamental science and medicine emerged. Myself, along with two other PKU alumni, proposed the idea to our alma mater, and in 2010, BIOPIC was officially established.

New BIOPIC building with expanded research facilities Credit: BIOPIC
What makes BIOPIC unique?
BIOPIC is a technology-driven biomedical research centre, fusing basic research, biotechnology development, and medical applications. I believe breakthroughs in research tools are essential for driving life science research. Cross-disciplinary integration is also vital. To this end, we’ve brought in multidisciplinary researchers, including physical chemists or biophysicists, such as myself, structural biologists, molecular biologists, as well as those working on biotechnology or engineering, mathematics, and computational science. Many of our new biotechnologies are results of collaboration. I’d say the level of interdisciplinarity at BIOPIC is unparalleled.
We are also unique in our emphasis on clinical translation. We have works published in leading journals, but that is not our ultimate goal. We encourage our researchers to commercialize their technologies, and to collaborate with clinicians for applications in medicine. As scientists, we are interested in advancing fundamental science, but are also keen to benefit society with our technologies.
What are the cutting-edge technologies that BIOPIC focuses on?
BIOPIC was initially named Biodynamic Optical Imaging Centre, based on my earlier work at Harvard on single-molecule imaging. Now we’ve broadened our scope. We focus on single-cell genomics technologies, including high-throughput sequencing, genetic editing, and microfluidic control. These, along with single-molecular imaging, super-resolution and label-free imaging technologies, enhanced by big data analytics can be used to advance our basic science research, ranging from genomics, genetics and molecular biology, to developmental biology, tumour immunology and bioinformatics. We also emphasize clinical translation of our technologies. A whole-genome amplification technique that we developed enables high-coverage sequencing, and has been used in pre-implantation genetic screening. Our error-correction code sequencing, CRISPR-based sequencing, along with a safe and more efficient gene-editing tool have been used in analysing human embryonic development, stem cells, and tumour microenvironment, informing reproductive medicine, and the diagnosis and treatment of cancer and infectious diseases.
Why are you focusing on single-cell genomics?
Single-molecule technologies have enabled us to explain life processes at the molecular level, an important development in the 20th century. Single-cell genomics is a next-generation sequencing technology with potential to bring in an even bigger revolution, I believe, with greater impact than that of the Human Genome Project. It allows us to probe the full complement of DNA, as well as RNA existing within a cell, mapping genome structure and function. It helps us to answer questions like how genetic information carried by DNA or RNA controls cell functions. This will generate new insights into some fundamental life science issues, and bring new possibilities for clinical application, including prenatal genetic testing and cancer diagnostics.
To find its footing in this rapidly moving field, it is important for China to develop single-cell genomics technologies, including its own sequencers. And we’re driving such technological developments at BIOPIC.
How do single-cell technologies pave the way for precision medicine?
Precision medicine must take into account individual variability in genes, and the genetic understanding of diseases enabled by single-cell genomics helps doctors to identify the most appropriate treatment for individuals. In cancer treatment, for example, using next-generation sequencing technologies, we can analyse individual tumour cells and their microenvironment, facilitating identifying new targets for therapy. Recently, one of our PIs revealed the dynamics of single immune cells in liver cancer, using single-cell RNA sequencing, shedding light on potential treatment strategies.
Another example is our collaboration with Jie Qiao, from Peking University Third Hospital, to improve pre-implantation genetic diagnosis and screening. Based on the single-cell whole-genome amplification technology we developed earlier, our new approach has been used to select, with high precision, healthy embryos from couples with known monogenic diseases.
How does BIOPIC promote research translation?
BIOPIC is all about translating technologies into applications — we are only limited by our own imagination. A healthy and fluid ecosystem is needed for translational research, and at PKU, we have abundant resources and strengths in a variety of fundamental fields. On the clinical side, we have many affiliated hospitals. We are also close enough to the bio hub at Zhongguancun Science Park, home to biotech giants (such as BeiGene) and start-ups alike. In fact, five out of the six biotech companies founded by BIOPIC researchers are registered in Beijing. BIOPIC provides our scientists with enough support and freedom, so that they dare to dream big, while nurturing a close-knit intra- and extramural community, so their synergy can spark ideas to translate into clinical contributions.
What is your expectation for BIOPIC?
We aim to make BIOPIC a world-leading research institution in biomedicine, pioneering in cutting-edge biotechnologies and original research. We develop and harness innovative bioimaging and genetic sequencing techniques, which provide tools to investigate basic life science and medical research questions at the molecular and cellular levels. I also expect that our work will be translated into technological and medical breakthroughs, ultimately benefiting society and mankind.
High precision for reproductive science
Researchers at BIOPIC have worked on reproductive technologies that help reduce the likelihood of passing on genetic disorders.

Xie (left), Qiao (second left), and Tang (right), together with the IVF baby screened by MALBAC for genetic diseasesCredit: BIOPIC
For couples with infertility issues, particularly those with genetic defects, in vitro fertilization (IVF), along with pre-implantation genetic diagnosis (PGD) increase the chances of having healthy babies. These advances are brought by next-generation sequencing.
Harnessing single-cell genomics, researchers at PKU’s BIOPIC, in collaboration with physicians from Peking University Third Hospital (PUTH), have developed a highly accurate PGD method that can simultaneously screen for monogenetic diseases and chromosome abnormalities.
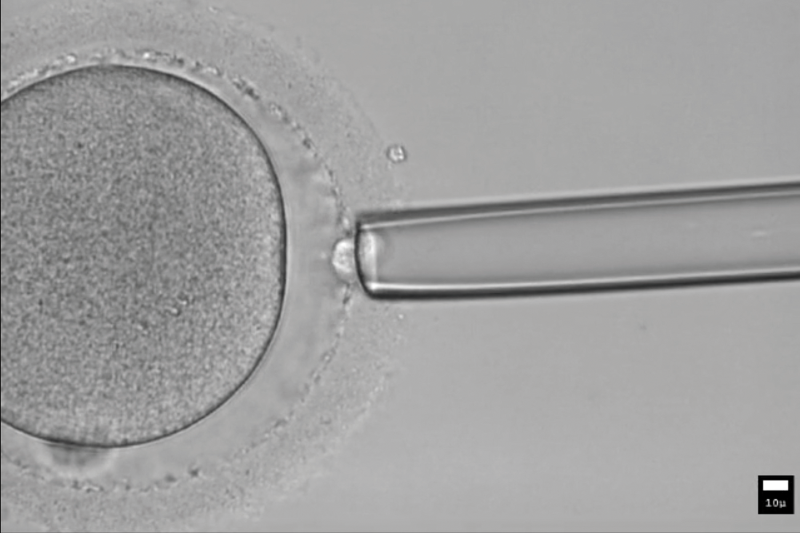
MALBAC can be used to find variations in gene-copy number as well as point mutations.Credit: BIOPIC
Revolutionizing prenatal screening
BIOPIC’s method is based on a whole-genome amplification technique developed by a team led by Xiaoliang Sunney Xie, BIOPIC’s director. It is not possible to read the entire genome of one single cell. Whole genome amplification is needed to generate enough DNA replicates for sequencing. However, the traditional technique leaves a large fraction of the genome undetectable, hindering accuracy.
Xie’s multiple annealing and looping-based amplification cycles (MALBAC) strategy enables sequencing for more than 90% of a human cell’s genome, and can be used to find variations in gene-copy number and chromosomal abnormalities.
Another notable error in PGD is failure to detect single-nucleotide variations (SNVs), which can lead to the selection of the wrong embryo. This can be circumvented by linkage analyses. However, traditionally, separate procedures for the same embryo are required to detect SNVs, and copy number variation and aneuploidy, which are chromosome abnormalities.
Xie’s team, together with BIOPIC’s Fuchou Tang group, and PUTH’s Jie Qiao group, collaboratively developed a method called the ‘mutated allele revealed by sequencing with aneuploidy and linkage analyses’ (MARSALA), which, carried out with MALBAC, enables simultaneous identification of mutational and chromosomal defects, all using the same embryo sample. This offers a cost-effective approach to PGD, improving precision, and streamlining the process.
MARSALA was used at PUTH to select healthy embryos from couples with known monogenic diseases. In 2014, two IVF babies were born without their parents’ monogenic diseases, based on this embryo selection technique. Since then, it has been used to help more than thousand disease couples to have healthy babies.
For Xie, this is a tangible example of the benefits of precision medicine. “I’m delighted that our research can make some contribution to medicine,” Xie said when he was awarded the Albany Medical Center Prize in Medicine and Biomedical Research in 2015 for his development of the MALBAC technology, which also has application in cancer research.
Understanding embryonic development
Use of single-cell technologies to address reproduction issues is also demonstrated in a BIOPIC study that explores the molecular mechanisms regulating the implantation of human embryos, a failure of which can lead to early pregnancy loss. However, given the difficulty of studying human embryos in vivo soon after implantation, it is unclear how gene regulatory network and epigenetic mechanisms control this key process in reproduction.
By combining an in vitro culture system and single-cell multi-omics sequencing, Tang developed an approach capable of analysing more than 8,000 individual cells from embryos after implantation. With Qiao, they reconstructed the implantation process by profiling transcriptome and DNA methylome, and revealed the dynamics of DNA methylation, which plays key roles in the epigenetic regulation of embryo development. “The insights we gained will help advance future efforts on reproductive medicine,” says Tang.
Tang’s team is also recognized for pioneering single-cell DNA methylome sequencing in 2013, a breakthrough in single-cell epigenomics. Based on the technologies, they have revealed many mechanisms about DNA methylation, improving understanding about human germline cell development.
A detailed view of disease mechanisms
Using single-cell technologies, BIOPIC researchers have improved disease treatment.
Xie’s single-cell technologies locate neutralizing antibodies against SARS-CoV-2 to block its entry into host cells.Credit: DEM10/GETTY
Advances in single- cell technologies are shaping the future of biology. New methods have allowed researchers to gather and analyse information on genome structure and functions from individual cells, one at a time, providing new insights into diseases. Harnessing single-cell technologies, researchers at BIOPIC have revealed the mechanisms of some of the most devastating diseases, from cancer to infectious diseases, informing treatment strategies.
Single-cell RNA sequencing has enabled Zhang’s team to simultaneously analyse more than 5,000 single T cells.Credit: STEVE GSCHMEISSNER/SCIENCE PHOTO LIBRARY/GETTY
Understanding tumour microenvironment
Tumour immune microenvironment affects tumour initiation and response to therapy. However, this has been poorly understood, particularly for liver cancer. A team led by BIOPIC researcher, Zemin Zhang, has characterized T cells in this common cancer, showing the tumour microenvironment immune landscape at the single cell level.
“We want to use the most detailed method to describe the compositions, functions, and dynamic changes and relationships of various types of cells in cancer,” says Zhang. “This will facilitate molecular typing of cancer and devising treatment strategies for patients.”
Single-cell RNA sequencing has enabled Zhang’s team to simultaneously analyse more than 5,000 single T cells, isolated from peripheral blood, tumour, or normal tissues, revealing their distinct functional composition, along with their transcriptomes. Using the data, researchers identified 11 T cell subsets, along with their signature genes, based on molecular and functional properties, and outlined their developmental trajectories. Particularly, they found that many of the T cells in tumours are in a state of dysfunction, allowing tumours to evade immune surveillance.
More recently, combining two single-cell RNA sequencing technologies, the team extended their exploration to broader immune cells from various tissues in liver cancer patients. Here, they systematically compared their profiles, traced the dynamics of immune cells in liver cancer, including how they develop, interact, and migrate, adding new dimensions to the tumour immune landscape.
Zhang expects biological insights provided by his research to help identify novel therapeutic targets, as well as biomarkers for studying responses to current immunotherapies.
Seeking COVID-19 treatment
Single-cell technologies are also being harnessed in an effort to treat COVID-19. “Our strategy was to find neutralizing antibodies against SARS-CoV-2 from the blood of recovering COVID-19 patients, which could block virus entry into host cells,” says Xiaoliang Sunney Xie, BIOPIC’s director.
High-throughput single-cell sequencing techniques have significantly accelerated the work of Xie’s group. In a July Cell paper, they reported finding 14 potent neutralizing antibodies by sequencing B cells from 60 recovering patients. One antibody, called BD-368-2, exhibited particularly high therapeutic and prevention efficacy in mouse models.
To better understand how neutralizing antibodies work, the team further explored the molecular mechanisms of BD-368-2. Xie’s colleagues, Xiaodong Su and Junyu Xiao, revealed its high-resolution structure, and showed that BD-368-2 blocks recognition by ACE2, the protein acting as the receptor for the SARS-CoV-2 virus, by occupying all three receptor-binding domains simultaneously. They also found another antibody that, when paired with BD-368-2, can prevent escape from neutralizing antibodies by the virus spike protein variants, a cause for drug resistance.
“We are excited about the therapeutic potential of our neutralizing antibody candidate,” says Xie. “It’s always my goal to find clinical application of our single-cell technologies.”
Improving the accuracy of genetic sequencing
A new genetic sequencing technology developed by BIOPIC researchers has improved ways to analyse DNA.

Huang’s group has explored more than 30 fluorophores to find the ones with the brightest and longest lasting signals when combined with nucleotides.Credit: DON FARRALL/GETTY
DNA sequencing is an indispensable laboratory technique under rapid development. It unlocks genetic information carried in DNA segments, the quality of which relies on the level of sensitivity, accuracy and speed of sequencing technology. Eliminating errors in DNA sequencing has proved one of the biggest challenges in the field.
Yanyi Huang’s laboratory at PKU’s BIOPIC is committed to advancing technologies to facilitate genome sequencing and its applications. The team has developed a new strategy to achieve ultra-accurate sequencing, which could be part of the ‘next-generation’ of DNA sequencing, according to Huang.
Named error-correction code (ECC) sequencing, Huang’s method builds on an emerging technology called fluorogenic sequencing, a sequencing-by-synthesis method that adds, one at a time, nucleotides labelled with a dye into microwells that contain target DNA fragments. When the nucleotide is incorporated into new DNA strands, the dye becomes fluorescent, and the intensity of the signal makes detection possible.
Each sequencing chemistry has its pros and cons, and imperfections lead to errors when deducing sequences from the signals detected during sequencing reactions. At present, all ‘next-generation sequencing’ methods have higher error rates than the first-generation Sanger sequencing approach.
In ECC sequencing, Huang’s team adjusts the way nucleotides are added, resulting in extra information for each DNA fragment sequenced. Drawing analogies with the encoding and decoding strategies used in information communication for error detection and correction, Huang’s team has embedded information redundancy in sequencing reads. The key is a specially designed algorithm, which combines the results and acts like a ‘course corrector’, identifying and fixing any errors.
The team has built a laboratory prototype to perform the ECC experiments and found out that it can generate error-free sequences up to 200 base pairs long, with raw accuracy reaching more than 98%, as reported in their 2017 Nature Biotechnology paper.
“We are glad to have developed an elegant way that enables accurate identification of extremely rare genomic variations,” Huang says. Huang’s group has been investigating the potential of ECC sequencing for nearly a decade, having explored more than 30 fluorophores — chemical compounds that produce fluorescent signals when activated — to find the ones with the brightest and longest lasting signals when combined with nucleotides.
To perform ECC sequencing on a larger scale and to make it more user-friendly, Huang is leading his team to develop an automated microfluidic device and prototype.
Early data show comparable results that could place ECC sequencing on equal footing with available commercial sequencing technologies. Huang is confident that ECC sequencing could be a potent tool in precision medicine, including detecting genetic mutations in tumour tissues.
Leveraging learning in gene editing
Developments in gene editing technologies have improved high-throughput screening for disease studies.
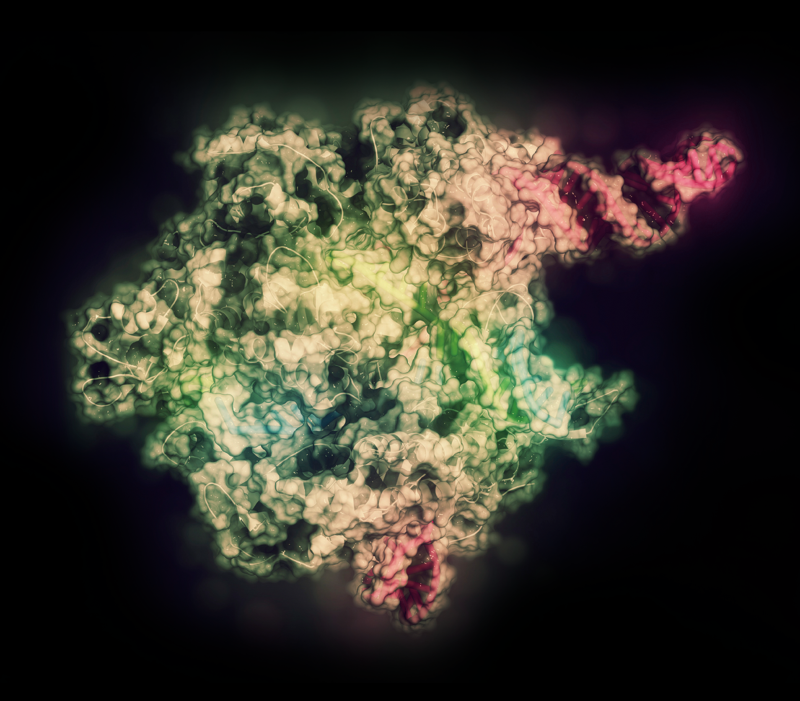
CRISPR-CAS9 gene editing complex (pictured) features more efficient and precise edits and modifications compared to conventional gene editing.Credit: MOLEKUUL/SCIENCE PHOTO LIBRARY/GETTY
Gene editing is at the centre of the next generation of biotechnology. By changing the messages encoded within DNA sequences, it creates new possibilities for researchers to unravel questions surrounding the inner workings of genes and their functions. It has ignited a revolution in life sciences research, with applications in healthcare and agriculture.
At PKU’s BIOPIC, a team led by Wensheng Wei, also a professor at PKU’s School of Life Sciences, is pushing frontiers in gene editing and functional genomics. They have made significant developments in large-scale high-throughput screening in genomes.
Wensheng Wei and his team are pushing frontiers in gene editing and functional genomics.Credit: KEITH CHAMBERS/SCIENCE PHOTO LIBRARY/GETTY
Conventional gene editing works on a single gene at a time. Wei has been trying to identify gene functions and to edit them in a high-throughput fashion, and has turned to the CRISPR-Cas9 system, which enables modifying and deleting sections of DNA efficiently and precisely. Using CRISPR technology as a basis, his team has established a suite of new high-throughput functional screening methods, improving the accuracy and efficiency of gene screening.
The team was one of the first to establish a high-throughput CRISPR screening technology for protein-coding genes. Soon after, they developed CRISPR screening methods for long non-coding RNAs, which enable efficient systematic deletion of large fragments of genetic information. It was used for identifying a non-coding RNA that regulates cancer cell proliferation, and later, updated to allow for more targeted gene screening and for use in an entire genome.
These advances have paved the way for studying pathogenesis of cancer and various infections. An example is the discovery of a host cell receptor linked to enteroviruses, such as the echovirus, which, in rare cases, can cause life-threatening inflammation of the brain. The finding has brought a deeper understanding of how this infection occurs and informed treatment strategies.
Wei’s team is also working on a new gene editing technology that could potentially rival CRISPR-Cas9. Reported in a 2019 Nature Biotechnology paper, this technology, called ‘leveraging endogenous ADAR for programmable editing of RNA’, or LEAPER, can be a safe gene-editing tool, according to Wei. “It offers a new tool for basic life sciences studies and development of therapeutics.”
CRISPR-Cas9 harnesses a naturally occurring genome editing system in bacteria. At the core is the Cas9 enzyme that can target, cut, or modify specific DNA sequences. However, a major limitation for its clinical use is its reliance on Cas9, which, as a foreign protein, can trigger the body’s immune responses.
LEAPER uses engineered RNAs that can recruit ADAR enzymes already naturally occurring in cells, thus, minimizing side effects. Highly specific, it is more streamlined than CRISPR-Cas9. Lab tests showed that LEAPER’s editing efficiency reached up to 80% in human lung fibroblasts, bronchial epithelial and immune cells.
LEAPER can also be used to repair deficient cells. In laboratory conditions, it was used to restore catalytic activity of cells from patients with Hurler Syndrome, an inherited condition that impairs a body’s ability to digest sugar.
“We are confident about the broad application potential of this precise and efficient RNA editing technique,” said Wei.
The effect of working in concert
a collaborative study on DNA-protein interactions exemplifies the multidisciplinary and cooperative research at BIOPIC, laying the foundation for biotechnology breakthroughs.
Improving understanding of biological systems, including the functions of DNA in cells, is essential for researchers working on drug discovery or disease mechanisms. A fundamental life sciences question with significant implications for regulation of general biological functions has to do with allostery, where the binding of a molecule on one site of a macromolecule affects the binding of another molecule at a distant site.
Allosteric effects were conventionally found in proteins, such as enzymes. But, whether the binding of a protein is affected by the presence of another DNA-bound protein nearby was not known until a multidisciplinary group of BIOPIC researchers started probing the question.
Physical chemists, Xiaoliang Sunney Xie and Yujie Sun, have collaborated with structural biologist, Xiao-Dong Su, mathematical modelling experts, Hao Ge and Yi-Qin Gao, and others, to systematically explore allosteric effects in DNA. They proved, for the first time, the allosteric effect in DNA — protein interactions — and its universality for many protein pairs.
The discovery of this new physical property of DNA, published in Science in 2013, sheds light on methods for genetic regulation or control of gene expression.
Their study started on the measurement of how long a protein binds on its DNA-binding site before falling off. They labelled the protein with a fluorescent dye, and used single-molecule fluorescence microscopy for the experiment, which enabled monitoring protein detachment events from a few hundred DNA templates simultaneously.
The experiment showed that two DNA-binding proteins can affect each other’s binding affinity to DNA, and that this effect varies by the distance between the two protein-binding sites, as the protein dissociation rate varies by distance. By analysing the patterns of how the dissociation rates change, they could also link the allosteric effect to the double-stranded structure of DNA.
Xie explained the mechanism underlying their discovery. “Think of the double-stranded DNA as two ropes, and the two DNA-bound proteins as monkeys with an arm on each rope, trying to hold their grip,” said Xie. “We found that the time a monkey is able to stay on the rope is affected by the presence of another monkey hanging nearby.”
This is because when one monkey widens their arm spread, working against the bond between the two stranded ropes, the other monkey nearby does not have to work as hard to stay in its place. But, if the other monkey is at a place where the gap between the two ropes is narrowed, it will have difficulty staying put. This explains how the binding of a protein on DNA is stabilized or destabilized by the binding of another protein, depending on their distance.
The team conducted well-designed control experiments to verify that the affinity change does come from DNA allostery, not from protein effects.
Their molecular dynamic simulations further showed that deformation of the double-helix structure is the origin of DNA allostery.
These findings have physiological relevance, as DNA allostery is found to affect gene expression in live cells and on transcription factor binding near nucleosomes.
The discovery is the result of concerted efforts, involving innovative experiment design, robust modelling, and single molecule imaging technology for analysing protein/DNA structures. “We are very grateful to have a multidisciplinary team in BIOPIC” said Su.
A DECADE OF BIOMEDICAL INNOVATION
With an open environment for interdisciplinary growth, BIOPIC is nurturing a generation of scientists who take on world-leading biomedical research.

Xiaoliang Sunney Xie (centre) and BIOPIC teamCredit: BIOPIC
From single-molecule imaging to next-generation sequencing and gene editing, rapid advancement in biotechnologies has given scientists unprecedented tools to explore the secrets of life. BIOPIC, a technology-driven biomedical research centre at PKU is dedicated to both developing and applying the most advanced bioimaging and sequencing techniques to address fundamental biological and medical problems.
In just 10 years, BIOPIC has become globally recognized for its pioneering innovations, leading in single-molecular dynamic imaging, single-cell high-throughput sequencing, high-efficiency gene editing, and so forth. Underlying its success is a collaborative spirit, according to Xiaoliang Sunney Xie, BIOPIC’s founding director. “We do not conduct investigations in a vacuum, but explore scientific questions in synergy,” he says. “Cross-disciplinary collaboration has always been the force behind biology’s ever-expanding frontiers.”
Fostering interdisciplinary synergy
Within BIOPIC, Xie and his team of talented principal investigators (PIs) form a multidisciplinary group. While each lab remains highly independent, with complete freedom to define its own researchers collaborate on several interconnected research themes, promoting mutual growth.
Error-correction code sequencing, for example, took seven years to develop from concept to laboratory prototyping. Using a specially designed algorithm, it enables error-free sequences on a large scale. The project was led by Yanyi Huang, whose research background ranges from chemistry, optics, to molecular biology. “It’s fun when we can work together to achieve otherwise impossible results,” Huang says. “It inspires even more meaningful collaborations beyond that of BIOPIC.”
Such collaborations have led to medical successes from the birth of the first MALBAC baby in 2014 to the rapid identification of neutralizing antibodies against SARS-CoV-2 in 2020. The centre is continually pushing for original advances in the science and technology of bioimaging, sequencing and gene editing. At the crossroads of these is microfluidic control and analysis of single cells, which incorporates microfluidic technologies with high-throughput studies of real-time microscopy, single cell genomics, and digital transcriptomics.
BIOPIC has also made significant impact to the biotechnology industry through its innovations. Successful technological transfers have been instrumental in the founding of six biotech startups by BIOPIC researchers, some of which have shown rapid growth and are now internationally recognized.
Institutionally, BIOPIC has established an international scientific advisory board of distinguished scientists, as part of its comprehensive and rigorous funding and advising processes. Peer evaluation is grounded in the impact and quality of research contributions, rather than mere metrics. Those who make the cut are provided with long-term research support, according to Fuchou Tang, who, like Huang, is also one of BIOPIC’s first PIs, having returned to China in 2010 from abroad. “We can afford to take on nascent, yet high-risk fields of study, and make mistakes,” says Tang.
BIOPIC’s diverse and open environment, cutting-edge research opportunities, and state-of-the-art facilities also provide a fertile ground for a future generation of scientists. From undergraduates to postdocs, many young researchers have been trained here. Nearly 20 BIOPIC alumni are now faculty members, heading labs worldwide from PKU and Tsinghua University to University of California, Berkeley.
“BIOPIC is founded with the vision and philosophy of driving the advancement of life science through breakthroughs in technology,” says Xie. “We have kept to that vision in the past 10 years, and will continue breaking through barriers to advance scientific knowledge through the cross-integration of multiple disciplines.”



